Authors: Prof. Dr. A. Burkhardt1
Pathology Laboratory
Reutlingen Obere Wässere 3-7
72764 Reutlingen Germany
1 in collaboration with an international team of pathologists
PDF file is available:
autopsy-directions-revised
Background and introduction
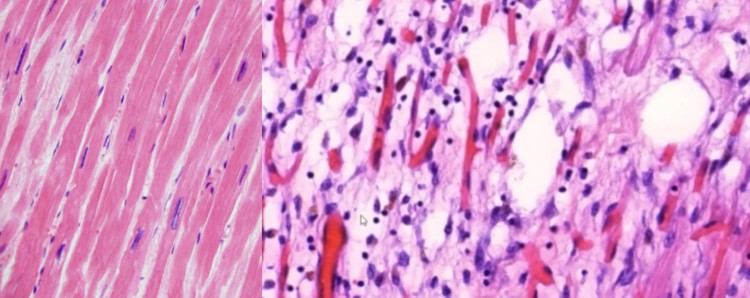
Dr. Burkhardt and colleagues recently carried out a series of 17 autopsies on persons deceased within days to months of vaccination. Initially, none of these deaths had been attributed to the vaccines. Nevertheless, Burkhardt and colleagues found characteristic lesions in multiple organs which led them to conclude that in most patients the vaccines were likely the cause of death. Key observations were widespread vasculitis with microthrombi as well as intense lymphocytic infiltration of multiple organs. A summary of these findings has been published before [1]. More recently, he and his colleagues have also demonstrated the expression of spike protein, induced by the vaccine, within the inflammatory lesions [2].
Here, Dr. Burkhardt gives guidance for conducting autopsies in similar circumstances. This document has been updated on .
1. Conduct of autopsies
Autopsies should focus on the following phenomena:
- thromboembolic events (both macro- and microthrombi)
- vasculitis
- myocarditis
- lymphocytic alveolitis
- peculiar inflammatory reactions (autoimmune reactions?)
- foreign material
1.1. Inspection of the body, sampling of injection site and lymph nodes
- Carefully inspect the entire integument, paying special attention to discoloration due to allergic-exanthematous reactions, e.g. brown coloring indicating hemosiderosis in the context of leucoclastic vasculitis
- Take tissue samples from the site of the vaccination (subcutaneous and muscle tissue)
- Preserve the axillary lymph nodes on the side of injection, as well as enlarged lymph nodes from any other site
- Check the veins of the lower legs for thrombi, and especially in bedridden persons also the plantar veins
1.2. Body cavities
Open up the three major body cavities according to standard practice. Take samples for histological examination from all organs and from any unusual lesions (infarctions, bleedings, thrombi etc.)
1.2.1. Thorax
- Check for thromboembolism by cutting open the major vessels
- Check the lungs for focal lesions
- Consider in-toto fixation of both lungs and preparation by serial section
- Take histological samples from the heart muscle in several different locations
- Optional: examine the heart’s excitation conduction system, especially in cases of sudden cardiac death. Pay special attention to the region of the atrioventricular node
1.2.2. Abdomen
- Pay special attention to the spleen (histology) and to Peyer’s Patches
- Cut open the liver veins all the way to the periphery in order to check for veno-occlusive disease
- Also examine the ovaries, which allegedly may contain deposits of foreign material
1.2.3. Brain, eyes, and ears
- Look for infarctions or bleedings. Pay special attention to the superior thalamostriate vein (vena terminalis)
- Preserve the hypophyseal gland
- If possible, carry out fixation in toto and subsequent neuropathological examination
- Critical: examine the eyes in case the deceased had been suffering from impaired vision (e.g. recently developed visual field defects)
- Examine the inner ear in patients with loss of hearing
1.3. Tissue sampling
Routine sampling from all organs, in addition to those specifically mentioned above:
1. Sample all recognizable lesions, especially thrombi, which should be preserved together with the surrounding vascular wall
2. Take samples from arteries even when they don’t contain any thrombi, especially from the following:
- aorta
- coronary arteries
- carotid arteries
- the Circle of Willis (circulus arteriosus cerebri)
- arteries of the leg
3. Take samples of striated muscle from at least two locations, always including the lower leg muscles
4. Sample the bone marrow in at least two different sites with active hemopoiesis
5. Take samples from the thyroid gland and from the salivary glands (look for autoimmune phenomena)
1.4. General considerations
- Photographically document all relevant changes and important normal findings
- Preserve organs until the histological samples have been assessed, for the purpose of possible further examinations
- When embedding of the histological samples, ensure compatibility with subsequent immunohistological or PCR investigations (virus fragments)
- If there is no significant autolysis yet, preserve samples for electron microscopy—search for virus particles or fragments, unusual materials etc.
2. Evaluation of organ samples from deceased or biopsies from living patients after COVID vaccination (microscopy, histology, immunohistochemistry)
In any case and on all organs:
- Search for birefringent material
- Stains: HE, PAS, iron
2.1. Immunohistochemical differentiation of inflammatory cells
In case of inflammation, further definition by immunohistochemistry, depending on the histological picture:
- CD 3 (T lymphocytes)
- CD 4 (T helper cells)
- CD 8 (cytotoxic T lymphocytes)
- CD 14 monocytes
- CD 20 B lymphocytes
- CD 56 cell adhesion (NK cells)
- CD 68 macrophages
- CD 31/D2-40 endothelium
- Complement deposits
2.2. Immunohistochemistry to detect vaccine-induced spike protein expression
- Use anti-SARS-COV-2 spike protein/S1 antibodies to test for presence of spike protein in tissue samples. Always include myocardium and spleen tissue samples
- If spike protein is detected, use anti-nucleocapsid antibody to examine expression of SARS-COV-2 nucleocapsid: presence of nucleocapsid indicates viral “breakthrough” infection, absence of nucleocapsid supports vaccine-induced spike protein expression
- Perform positive and negative controls using vaccine-transfected and non-transfected cell cultures
2.3. Unidentified foreign material, other unusual findings
If histological examination reveals unidentified foreign material, preserve tissue samples using conditions suitable for
- electron microscopy
- Raman microscopy
- X-ray and laser microanalysis
In case of any unusual findings, carry out fixation for electron microscopy if possible.
3. Further considerations and measures
If the examinations detailed above provide evidence suggestive of vaccine-induced death, consider the following steps:
- Preserve tissue samples of lesions, including the site of vaccine application
- Obtain the consent of relatives, and if applicable the court prosecutor, for carrying out paraffin embedding and histological sections (HE, PAS, FE) of all organs
- Depending on the findings, initiate further investigations by cooperating special laboratory or in a reference laboratory
References
- Bhakdi, S. and Burkhardt, A. (2021) On COVID vaccines: why they cannot work, and irrefutable evidence of their causative role in deaths after vaccination.
- Burkhardt, A. and Lang, W. (2022) First time detection of the vaccine spike protein in a person who died after vaccination against Covid-19.